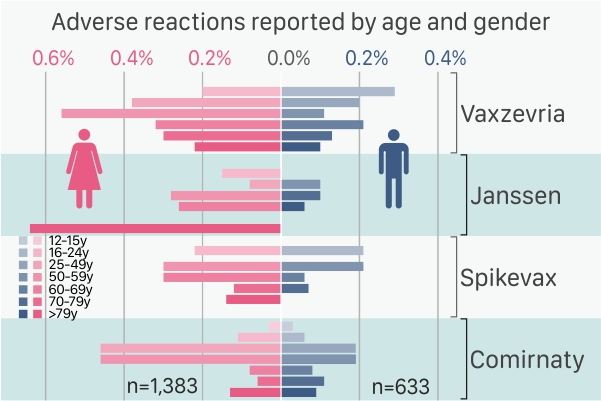
 Adverse reactions reported by age and gender;
Credit: Kangkan Halder
Adverse reactions reported by age and gender;
Credit: Kangkan Halder
In December 2021, Luxembourg's Ministry of Health reported a total of 2,016 suspected adverse reactions following COVID-19 vaccinations recorded up to Monday 29 November 2021.
A total of 917,463 doses of vaccine were administered to Luxembourg residents as of that date, corresponding to 456,942 people who received at least one dose of a COVID-19 vaccine.
The ministry issued a caution that the 'suspected adverse reactions' are to be understood as medical events which have been observed after vaccination but are not necessarily related to, or due to, the vaccine.
The suspected adverse reactions were recorded by the Pharmacy and Medicines Division of the Department of Health in close collaboration with the Regional Centre for Pharmacovigilance of Nancy (France).
Of the 917,463 vaccine doses administered, 688,620 (75%) doses were Comirnaty (Pfizer-BioNTech), 110,888 (12.1%) doses were Vaxzevria (AstraZeneca), 74,798 (8.2%) doses were Spikevax (Moderna) and remaining 43,157 (4.7%) were Janssen COVID-19 vaccines.
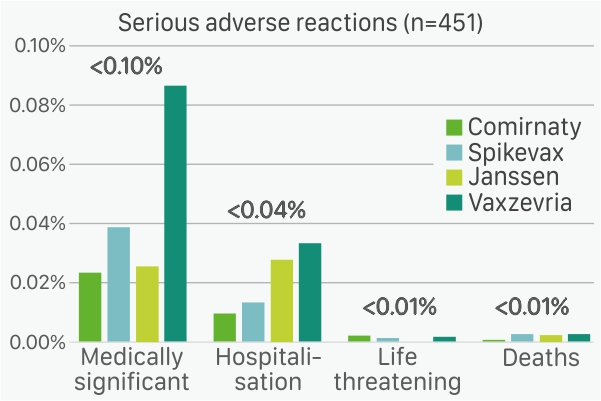
The main findings of the data analysis (see graph above) show less than 1% adverse reactions reported by age and gender for all four European Centre for Disease Prevention and Control (ECDC) approved vaccines. Moreover, severe adverse reactions were reported for less than 0.1% of doses administered for all four COVID-19 vaccines (see graph below).
The 2,016 adverse reactions represented 0.22% of all vaccine doses administered and, conversely, adverse side effects were not reported in relation to 99.78% of vaccinations.
Moreover, of the total 2,016 adverse reactions reported in Luxembourg, 1,565 (77.6%) were deemed not to be medically serious.
Methodology
For the first analysis, different numbers of people across different age-groups and gender received the vaccine doses (for each of the four vaccines) and therefore, adverse reactions were computed per 100 individuals in each group for each vaccine individually.
Similarly, for the second analysis, as 75% of all doses were Comirnaty (Pfizer-BioNTech) and so on (Vaxzevria, Spikevax and Janssen); the severe adverse reactions were computed per 100 doses of each vaccine manufacturer.
Of the 451 severe adverse reactions reported, 297 were medically significant cases (temporary incapacity for work or the inability to leave home due to symptoms such as fever, muscle pain, malaise, etc.), 125 cases needed hospitalisation, 18 cases were life-threatening and 11 cases resulted in death.
In terms of percentages, medically significant cases represented less than 0.1%, hospitalisations represented less than 0.04% and life threatening and deaths represented less than 0.01% of of each vaccine type administered.
In comparison, between 4 January and 28 November 2021, 2,482 hospitalisations were recorded in Luxembourg, equivalent to 0.39% of the resident population (vaccinated and unvaccinated included).
Similarly, 343 deaths were reported in Luxembourg in the above period compared to 11 deaths suspected of severe adverse effect of COVID-19 vaccines.








