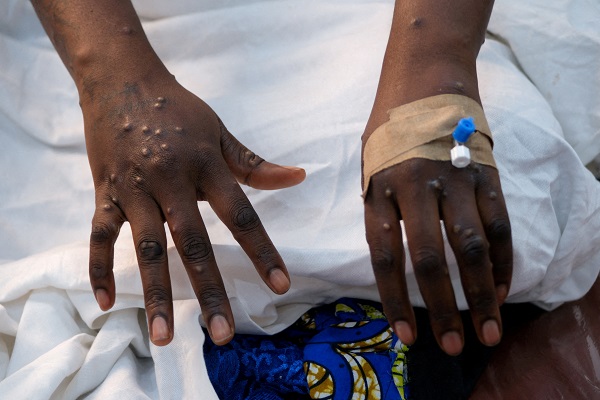
 The hands of a patient with skin rashes caused by the mpox virus are pictured at the treatment centre of Vijana Hospital in Kinshasa, Democratic Republic of Congo, 30 August 2024;
Credit: Reuters/Justin Makangara/File Photo
The hands of a patient with skin rashes caused by the mpox virus are pictured at the treatment centre of Vijana Hospital in Kinshasa, Democratic Republic of Congo, 30 August 2024;
Credit: Reuters/Justin Makangara/File Photo
(Reuters) - On Monday 14 October 2024, the World Health Organization (WHO) said it had approved Bavarian Nordic's mpox vaccine for adolescents aged twelve to seventeen years, an age group considered especially vulnerable to outbreaks of the disease that has triggered global concern.
The WHO said in a statement that it gave the Jynneos vaccine prequalification for adolescents on Tuesday 8 October 2024.
The WHO declared mpox a global public health emergency for the second time in two years in August after a new type of the virus spread from the Democratic Republic of Congo to its neighbours.
The United Nations agency approved the use of the vaccine in September as the first shot against mpox in adults, making it easier for badly hit African countries to access the vaccine.
Children, adolescents and those with weakened immune systems have been particularly vulnerable to mpox, a viral infection that typically causes flu-like symptoms and skin lesions filled with pus.
WHO's latest decision comes after the EU approved the drug for the vaccine for adolescents in September.
The Danish biotech firm is also preparing to conduct a clinical trial to assess the vaccine's safety in children aged two to twelve, potentially extending its use.
The trial, partially funded by the Coalition for Epidemic Preparedness Innovations, is expected to start in October.
The US Food and Drug Administration has also approved Bavarian's shot, but only for use in adults eighteen years and older, although it granted Emergency Use Authorization for its use in adolescents during the mpox outbreak of 2022.
Another mpox vaccine, LC16, made by Japan's KM Biologics, can already be given to children, according to the Japanese regulator, although it requires a special kind of needle.
Bavarian Nordic did not immediately respond to a request for comment on the prequalification.









